Thanks to Sofia D’Arena for collaborating on this article
On February 26, 2021, the Italian Medicines Agency (Agenzia Italiana del Farmaco, “AIFA”) provided further information on the new simplified pricing and reimbursement procedure introduced in October 2020 for generics and biosimilars. In particular, the AIFA provided clarification on cases of possible application of the automatic inclusion in Class C — without any negotiation step or prior classification in CNN — of a generic/biosimilar whose reference product (the “Originator”) is in Class C as well.
The above simplified procedure applies to medicinal products whose marketing authorization (“MA”) or line extension (“LE”) applications are submitted pursuant to Section 10, par. 1 (“generic application”), par. 3 (“hybrid application”), or par. 4 (“biosimilar application”) of Directive 2001/83/EC, implemented in Italy by Section 10, par. 1, 6, and 7 of Legislative Decree No. 219/2006, as amended from time to time.
Similarly, it also applies, to applications for Parallel Import Authorization. However, it does not apply to generic/hybrid/biosimilar medicines with therapeutic indications that go beyond those of the reference medicinal product.
The simplified procedure foresees, for the purposes of reimbursement, the classification of the abovementioned medicinal products in class C as soon as they are authorized, without prior placement in band “C Non-Negotiated” (“CNN”). As a result, it will not be necessary to further negotiate with the Health Technology Assessment (HTA) and Drug Economics Sector, nor with the Technical Scientific Committee (“Commissione Tecnico Scientifica, “CTS”)and the Pricing and Reimbursement Committee (Comitato Prezzi e Rimborso, “CPR”).
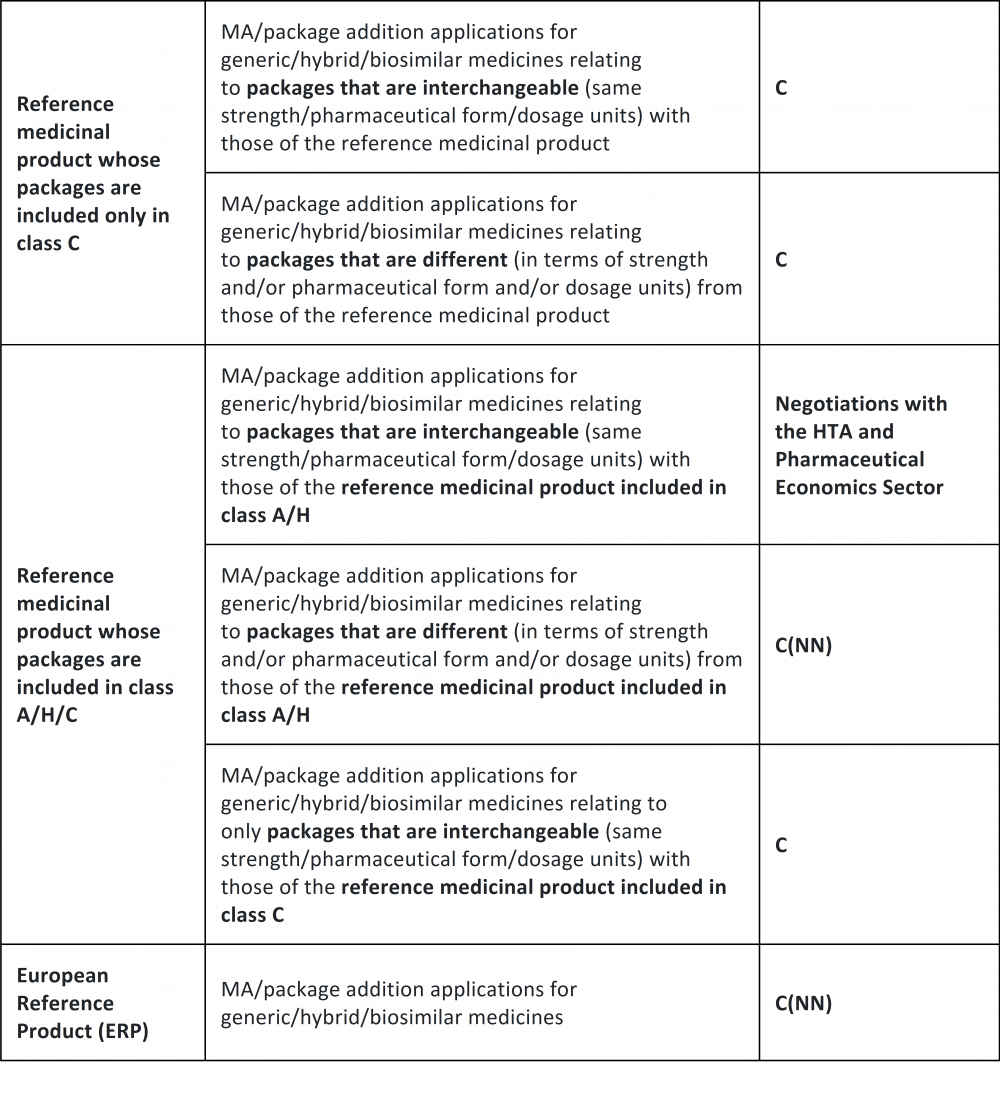
Therefore, whereas overlapping packaging means packages having the same quantity of active ingredient, pharmaceutical form, mode of administration, method of release, and dosage units, automatic classification in band C is foreseen for MA and LE applications concerning generic/hybrid/biosimilar medicinal products whose packages (i) overlap with that of an Originator that is entirely in class C; (ii) do not overlap with that of an Originator that is entirely in class C; (iii) overlap only with that of an Originator that is classified in band C, where the Originator also has different packaging reimbursed by the National Healthcare Service (band A/H).
Conversely, the new procedure does not apply to MA or LE applications concerning generic/hybrid/biosimilar medicinal products having one or more packages overlapping with those of the Originator that are reimbursed by the National Healthcare Service (Class A/H).
Please find below a table published on the AIFA website that summarizes the main cases of application of the new procedure.
The above rules will apply equally to new package applications resulting from type II or type I variations of generic/hybrid/biosimilar medicines.
In case of parallel importation applications (PIA), the packages of the medicines in question are interchangeable with those that have already been authorized for the reference medicinal product. Therefore, if the reference medicinal product is included in class C, PIA packages will automatically be included in the same class, without any prior inclusion in class CNN.
Timing for the application of the new procedure
As clarified by the AIFA, the new modalities for classification described above have been applied since the time of the CTS meeting of February 2021 (March 2021 for PIA).
As for previously authorized medicinal products, any application to pass from band CNN to band C must be submitted according to the existing procedures, since no retroactive mechanism applies.

